Atopia Therapeutics (Tx) is developing a first-in-class disease-modifying therapy for asthma and other allergic diseases.
This new treatment is based on a molecule from Helicobacter pylori, a bacterium present in the microbiome of 50% of the world’s population, which protects the host against allergic diseases by favoring a tolerant state of the immune system and thereby reduces inflammation.
About Us
Headquartered in Geneva, Switzerland, Atopia Therapeutics is backed by private investors.
Leadership

Grégoire Chevalier, Ph.D
CEO
Gregoire has over 10 years of experience in drug development and R&D, holding positions of Senior Scientific Officer at Institut Pasteur and Global R&D Program Leader at Enterome. He is an immunologist specialized in immuno-oncology, mucosal immunology and neuroimmunology.
Gregoire obtained and engineer degree in Agrobiosciences, a MSc in immunology and a Ph.D in immunology.

Jeffrey Shaw, Ph.D
COO
Jeff has over 20 years of experience in drug discovery, holding management and research positions at Glaxo, Serono and Merck-Serono.
He is a specialist in computer-assisted drug design for therapeutic proteins and small molecules, protein structural biology, enzymology, and protein chemistry.
Jeff obtained a MSc in organic chemistry, followed by a Ph.D in biochemistry, and postdoctoral fellowships at the University of Brandeis and the European Synchrotron Radiation Facility.

Christine Serratrice, MD, PD
CMO
Christine is a physician with a strong clinical background in internal medicine, with additional expertise in rare (lysosomal), auto-immune and inflammatory diseases.
Christine obtained her medical degree at the University of Aix-Marseille, followed by a Master’s degree in immunology and a Diplome for Advanced Studies in Management of Clinical Trials.

Joana Vitte, MD, Ph.D, Habil.
CSO
Joana is a physician and immunologist with a long-standing interest in the mechanisms of allergy, the interplay of infectious and allergic diseases, and their investigation at the epidemiological, clinical, and translational levels.
Challenge and Solution
Atopia Tx is addressing a growing unmet medical need in asthma and allergic diseases, in both adults and children.
Asthma and allergies represent an epidemic health issue, currently affecting 5% of the overall population. This prevalence is steadily increasing with the potential to affect 2 to 4 billion people worldwide by 2050. It is already the most common chronic non-communicable disease among children, with 1 in 10 children having asthma symptoms, and 1 in two having inadequately controlled symptoms.
population

by 2050

asthma symptoms
controlled symptoms

Global Asthma Report 2022
The incidence of H. pylori has dramatically decreased in developed countries and several developing areas in the past 50 years, and inversely correlates with the observed increase in incidence of asthma and other allergic disorders in children.
Kalach et. al. 2017
Solution
Atopia’s therapeutic solution, ATP-R13, is a patented, optimized, recombinant version of a protein secreted by Helicobacter pylori, a constituent of the gastric microbiome.
Atopia Tx is developing a formulated version of this immunomodulatory protein to be administered orally to patients, in order to re-equilibrate their immune system, and relieve inflammation and allergy symptoms. The treatment works irrespective of the antigen, which is a unique selling point.
Multiple in vitro and in vivo studies have already confirmed the molecule’s therapeutic potential. ATP-R13 has been administered orally, intraperitoneally, and intranasally to mice with no unexpected side effects. Chronic exposure to ATP-R13 and/or its variants has demonstrated its excellent tolerability and lack of toxicity in humans.
The lead optimization phase of the development program has been completed, and Atopia is currently initiating the IND-enabling activities of the program.
Atopia holds an exclusive world-wide licence from the University of Zurich and has filed a Composition of Matter patent in 2024 on the optimized clinical candidate.
Potential
Atopia has recently uncovered the mode of action of ATP-R13, (unpublished data). This MoA strongly suggests that ATP-R13 could be deployed against several indications of the atopic march, e.g. atopic dermatitis, allergic rhinitis and food allergy, as well as other indications such as IBD.
The prevalence of all possible indications is on the rise; with 5-year CAGR forecasts ranging from 7.5% (atopic dermatitis) to 27% (food allergy). The worldwide health costs caused by these indications together may exceed 55 billion USD in 2031.



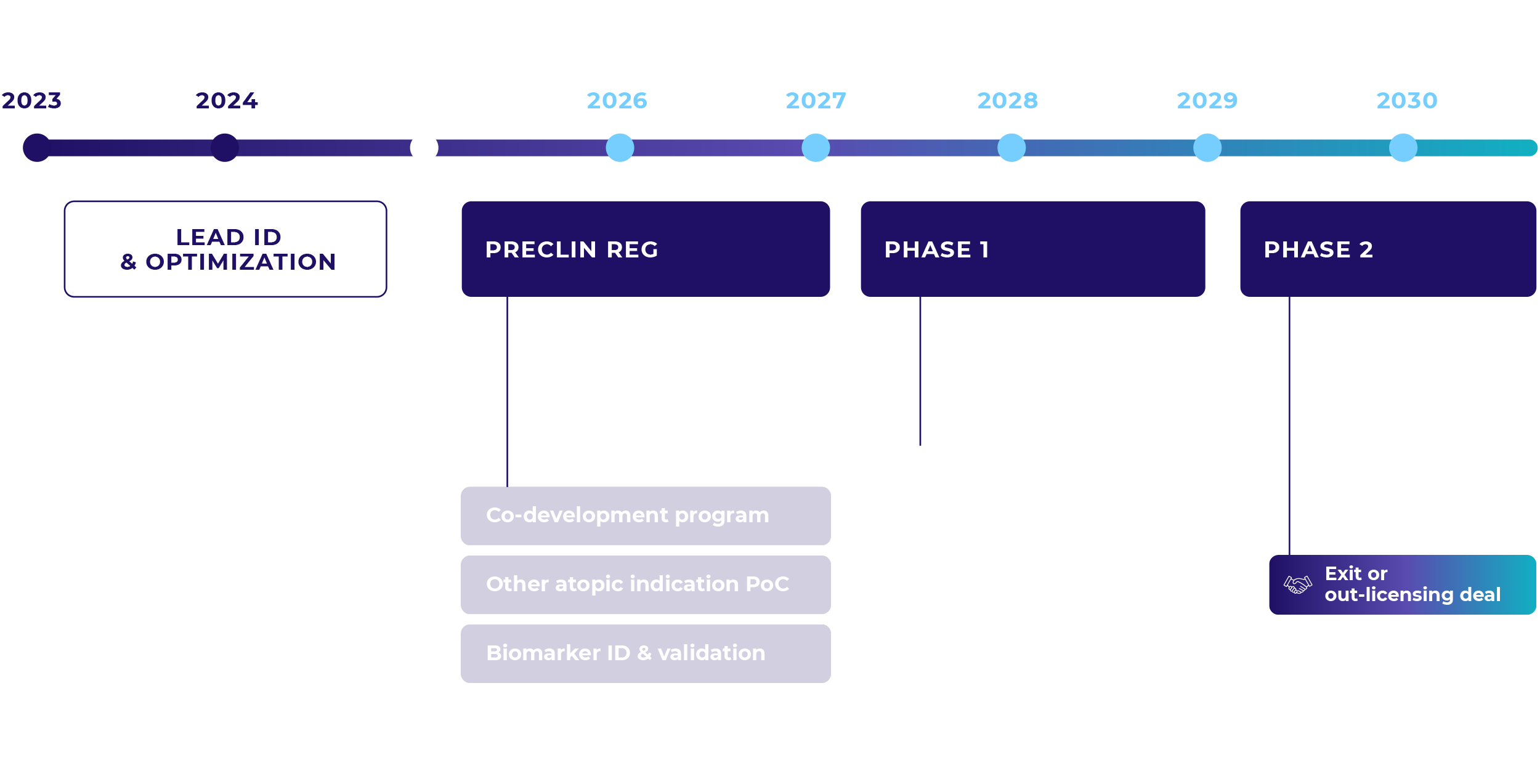
Atopia Path to Clinical Proof-of-Concept
News

May 7, 2024
Atopia Therapeutics, Formerly HpVac, Announces Appointment of Dr. Gregoire Chevalier as CEO and Business Update
Geneva (Switzerland), May 7, 2024 – Atopia Therapeutics, a leader in the development of breakthrough asthma and allergy therapies, today announced the appointment of Dr. Grégoire Chevalier as Chief Executive Officer (CEO). Formerly known as HpVac, the Company has repositioned itself under the new name Atopia Therapeutics to better reflect its commitment to addressing the growing global burden of atopic diseases.

March 27, 2023
HpVac reports positive effects of its lead compound HpVac-R13 in a murine model of allergic asthma following oral administration
Geneva, Switzerland, March 27, 2023 – HpVac SA, a company developing navel preventive and therapeutic first-line therapies against allergie and inflammatory diseases, today announced positive results of its lead compound HpVac-Rl3 on airway hyperresponsiveness and inflammation in a muri ne model of allergie asthma.

October 17, 2022
HpVac Announces New Animal Data of its Lead Compound Demonstrating Protection Against Allergic Asthma
Geneva, Switzerland, October 17, 2022 – HpVac SA, a company developing novel preventive and therapeutic first-line therapies against allergic and inflammatory diseases, today announced novel data on its lead compound HpVac-13 for the treatment and prevention of asthmatic attacks in an animal disease model of allergic asthma.
Join the mission
Become a Partner Today
Atopia Tx is currently open to collaboration with industrial and/or financial partners on the next phases of its development.
Partners will be able to rely on Atopia’s tested, solid expertise, based on over 20 years’ experience in business and pharmaceutical development, and on the company’s wide network of specialized consultants and CROs.
There are currently no open positions in the Atopia Tx team.



